Deadline date for switching
Patients currently on an originator drug for which there is a biosimilar version for their medical condition must switch to the biosimilar prior to the switch date in order to maintain coverage for the molecule through their Alberta government sponsored drug plan.
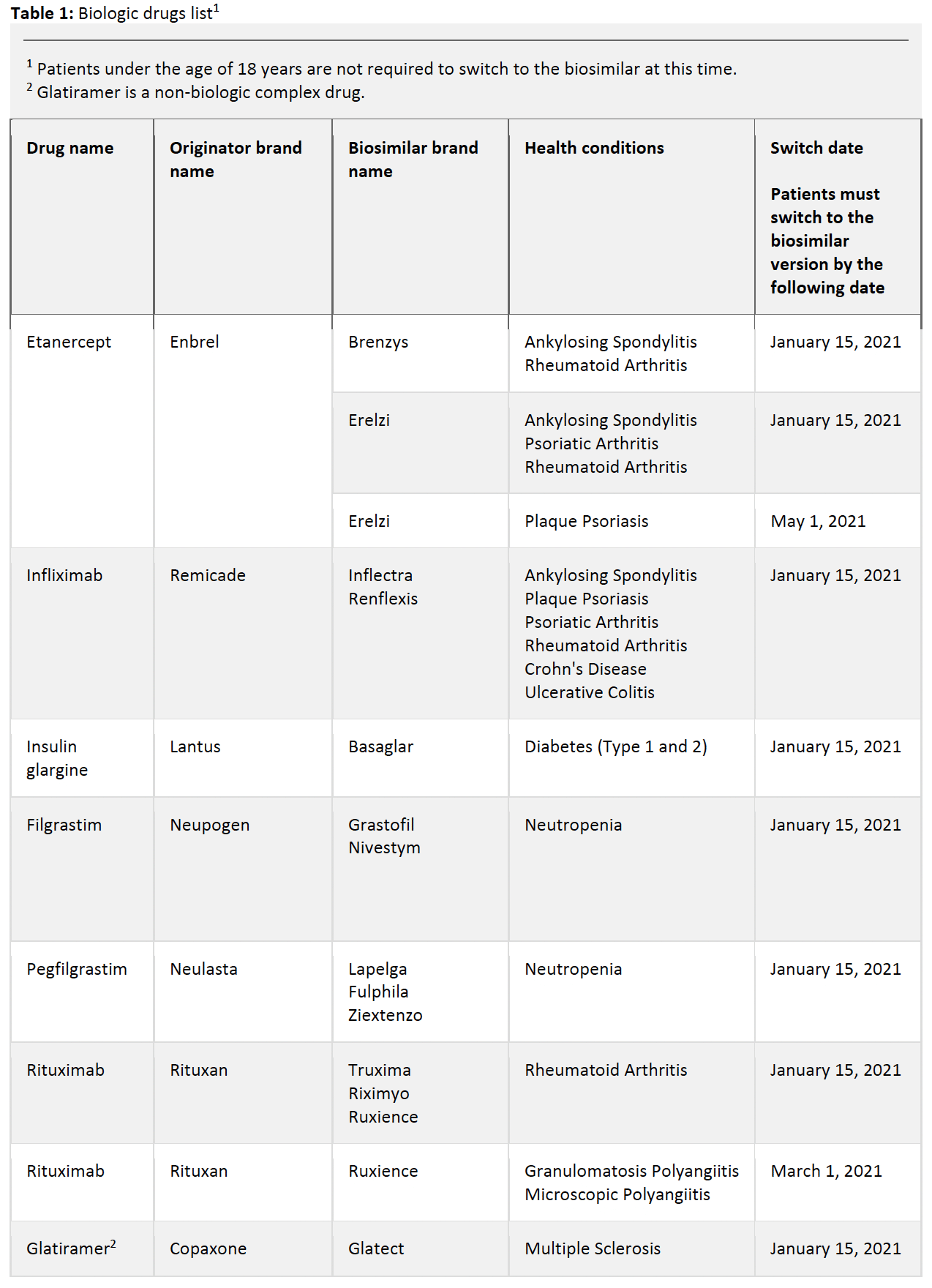
Please refer to the table below for a list of affected drugs and their designated switching dates.
Overview
A biosimilar drug is a highly similar version of the original biologic medication, known as an originator drug, but less expensive.
Alberta’s Biosimilar Initiative will expand the use of biosimilars by replacing the use of biologic drugs with their biosimilar versions whenever possible. This means patients will continue receiving the same safe and effective treatment, but at a lower cost.
Adult patients, except pregnant women, currently taking a biologic drug that has a biosimilar version for their medical condition must switch to the biosimilar drug prior to the switch date.
Under Alberta’s government sponsored drug plan, biologic drugs cost more than $238 million in the 2018-19 fiscal year, and are going up an average 16.2% per year. The originator biologic drugs Remicade, Humira and Enbrel are 3 of the top 4 drivers of drug spending in Alberta.
Switching to biosimilars will save between $227 million and $380 million over the next 4 years once fully implemented. These savings can be invested into other health care services for Albertans.
Biologic drugs affected
Only the biosimilar versions of the originator biologics listed below will be covered by Alberta government drug plans after the switch date.
Patients who are taking the originator biologics for the health conditions listed must switch to its biosimilar version of the drug.
Switching to a Biosimilar
Patients should contact their health care professional to discuss switching. Your health care professional can:
- answer questions about switching from an originator biologic drug to a biosimilar
- explain the process for switching
- discuss biosimilar options
- write a new prescription, if needed
- enrol you in a new patient support program, if needed
If you are unable to switch to the biosimilar version for medical reasons, your health care professional can help determine whether to request exceptional coverage of the originator biologic.
- Alberta Blue Cross biosimilar initiative patient information
- Alberta Blue Cross health professionals’ resources
Biologics and biosimilars
Biologics are drugs manufactured in, extracted from, or semi-synthesized from living cells through a highly complex manufacturing process.
A biosimilar is highly similar to the first biologic drug that was authorized for sale called an “originator biologic.” Due to the complexity of biologic drugs and the natural variability that results from using living cells, it is not possible for a biosimilar to be identical to its originator biologic drug, nor is it possible for different lots or “batches” of an originator biologic drug to be identical. These variations are not clinically meaningful.
Safety and effectiveness
The biosimilars included in Alberta’s Biosimilars Initiative are approved by Health Canada.
Health Canada reviews and approves all drugs before they can be sold in Canada. Biosimilar drugs undergo a rigorous review process and must demonstrate they are highly similar to the biologic drug.
Biosimilars have been used in Canada, Australia, the United States, the United Kingdom and the European Union for more than 10 years. Many European nations have required patients to switch to biosimilars under publicly funded drug plans.
Patients and health care professionals can have confidence that biosimilars are effective and safe for each of their authorized indications. Numerous research studies collectively show little to no difference in safety and efficacy when patients switch to a biosimilar.






